TOXLAB - Blog

All Blog
How To Register Medical Devices in India? Introduction Central Drug Standard Control Organization (CDSCO) has released the Medical Devices amendment rule and classification
Critical Points in OECD GLP Mutagenicity Studies Introduction Ensuring the quality and reliability of mutagenicity studies is crucial for evaluating the potential
Critical Points in OECD GLP Study Director Responsibilities Introduction The study director plays a critical role in ensuring the quality and integrity
Study Design of In Vitro Skin Absorption: Franz Cell Diffusion Assay (OECD 428) Introduction The Franz Cell Diffusion Assay (OECD 428) is a well-established
Test No. 301: Ready Biodegradability Introduction It’s actually not just one test, but a group of six related test guidelines for assessing
Introduction to Brazil’s Chemical Law (Law No. 15,022): The Brazil REACH Introduction On November 14, 2024, the President of Brazil sanctioned the
Acute Toxicity Studies – Study Design of OECD Guideline 402: Acute Dermal Toxicity Study in Rats Introduction The OECD Guideline 402 outlines
Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C) Introduction The Dermal Peptide Reactivity Assay (DPRA) is an in
The Unsung Guardians: Unveiling the World of Medical Device Testing Introduction Medical devices play a transformative role in healthcare, from simple bandages
Overview of 21 CFR 117 and Food Safety Regulations Introduction Title 21 of the Code of Federal Regulations (CFR), Part 117 (commonly
Unveiling the Truth: A Deep Dive into Dietary Supplement Testing Introduction Dietary supplements have become an integral part of modern life, promising
Acute Toxicity Studies – Study Design of OECD Guideline 425: Acute oral toxicity – up and down procedure Introduction The OECD Guideline
EFSA – Novel and Traditional Food Applications and Notifications: Regulations and Guidance as of 1st February 2025-What Will Change? Introduction The landscape
Master the Maze: Navigating the Design Control Process for Flawless Medical Devices Introduction In the world of medical devices, where even minor
Sensory Analysis : An Essential Tool For Product Development Introduction Sensory analysis, is a scientific discipline that evokes, measures, analyzes, and interprets
Critical Points in OECD GLP Mammalian Toxicology Studies Introduction OECD Good Laboratory Practice (GLP) guidelines provide a standardized framework for conducting and

Acute Toxicity Studies – Study Design of OECD Guideline 420: Acute Oral Toxicity – Fixed Dose Method
Acute Toxicity Studies – Study Design of OECD Guideline 420: Acute Oral Toxicity – Fixed Dose Method Introduction The OECD Guideline 420
Test No. 304A: Inherent Biodegradability in Soil Introduction The OECD 304 actually refers to two distinct test guidelines, both focused on assessing
Feed Testing: Safeguarding Animal Health and Performance Introduction In the intricate dance of animal agriculture, feed plays a pivotal role. It fuels
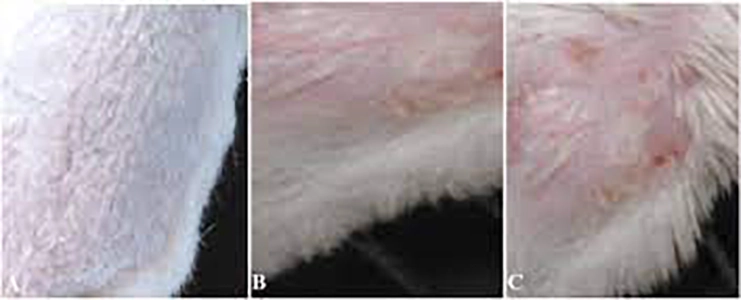
Acute Toxicity Studies – Study Design of OECD GLP TG 404 – Acute Dermal Irritation/Corrosion Study In Rabbits Introduction The Acute Dermal
Chemical Analysis: Understanding and Ensuring Quality Introduction Chemical analysis plays a crucial role in various sectors, from ensuring regulatory compliance to safeguarding
Labelling Requirements for Cosmetic Products under European Regulation 1223/2009 Introduction The European Union has established rigorous guidelines to ensure the safety and
Genetic Toxicology Studies – Study Design of Mammalian Bone Marrow Chromosomal Aberration Test (OECD 475) Introduction Mammalian Bone Marrow Chromosomal Aberration Test
Navigating the Complex Regulatory Landscape The Rise of Regulatory Complexity In today’s rapidly evolving business landscape, regulatory compliance has become a paramount
Is Your Brand Prepared for a Watershed Ban on HFSS (High in Fat, Sugar, and Salt) Food? Introduction The regulatory landscape surrounding
OECD GLP Series – Critical Points in OECD GLP TICO Responsibilities Introduction The Test Item Control Office (TICO) plays a crucial role






![All Blog 41 Study Design of In Vitro Skin Sensitization [Dermal Peptide Reactivity Assay (DPRA)] (OECD 442C)](https://www.toxlab.co/wp-content/uploads/2025/04/Study-Design-of-In-Vitro-Skin-Sensitization-Dermal-Peptide-Reactivity-Assay-DPRA-OECD-442C.webp)