TOXLAB - Blog

All Blog
The Crystal Clear Truth: Navigating the Intricacies of Water Testing Introduction Water, the very essence of life, sustains not just our bodies
Overcoming Challenges in Environmental Risk Assessment: Revisiting the OECD 309 Test Guideline Introduction Environmental risk assessment is a critical process used to
Test No. 202: Daphnia sp. Acute Immobilisation Test Introduction “OECD 202” actually refers to the Daphnia sp. Acute Immobilisation Test, a standard
Critical Points In OECD GLP Chemistry Studies Introduction Critical points in OECD GLP chemistry studies are those phases of the study that
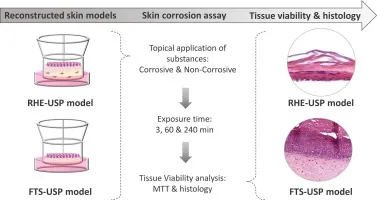
Study Design of EpiSkin-In Vitro Skin Corrosion (OECD 431) Introduction The EpiSkin-In Vitro Skin Corrosion Test, based on the OECD Guideline 431,
Genetic Toxicology Studies – Study Design of Mammalian Bone Marrow Chromosomal Aberration Test (OECD 475) Introduction Mammalian Bone Marrow Chromosomal Aberration Test
Safeguarding Our Food: The Crucial Role of Agrochemicals and Pesticides Testing Introduction The verdant fields and abundant harvests that grace our tables
Acute Toxicity Studies – Study Design of OECD 423 Acute Oral Introduction The OECD 423 Acute Oral Toxicity Test Guideline is a
Critical Points in OECD GLP Study Director Responsibilities Introduction The study director plays a critical role in ensuring the quality and integrity
Test No. 306: Biodegradability in Seawater Introduction The OECD 306 Guideline describes two methods for assessing the biodegradability of organic materials in
Food Testing: Ensuring Safe and Delicious Bites Introduction Food is the fuel that keeps us going, but it can also be a
Study Design of In Vitro Skin Sensitization – ARE-Nrf2 Luciferase Test Method [KeratinoSens] (OECD 442D) Introduction The KeratinoSens assay is a cell-based
Critical Points in OECD GLP Eco-toxicity Studies Introduction The Organisation for Economic Co-operation and Development (OECD) has developed Good Laboratory Practice (GLP)
Genetic Toxicology Studies – Study Design of In Vitro Mammalian Cell Gene Mutation Test Using the Hprt and xprt genes (OECD 476)
Genetic Toxicology Studies – Study Design of In Vitro Mammalian Cell Micronucleus Test (Human Peripheral Blood Lymphocytes-HPBL) (OECD 487) Introduction The in
Introduction to Brazil’s Chemical Law (Law No. 15,022): The Brazil REACH Introduction On November 14, 2024, the President of Brazil sanctioned the
Study Design of In Vitro Eye Irritation EpiOcular (OECD 492) Study Design Introduction The EpiOcular™ Eye Irritation Test (OECD 492) is an
Causality Assessment in Pharmacovigilance: Tools and Methods Understanding the Link Between Drugs and Adverse Events Pharmacovigilance, the process of monitoring drug safety,
OECD GLP Series – Critical Points in OECD GLP TICO Responsibilities Introduction The Test Item Control Office (TICO) plays a crucial role
The Medical Device Industry and SDLC: Essential Deliverables and Compliance Introduction The medical device industry is governed by strict regulatory standards to
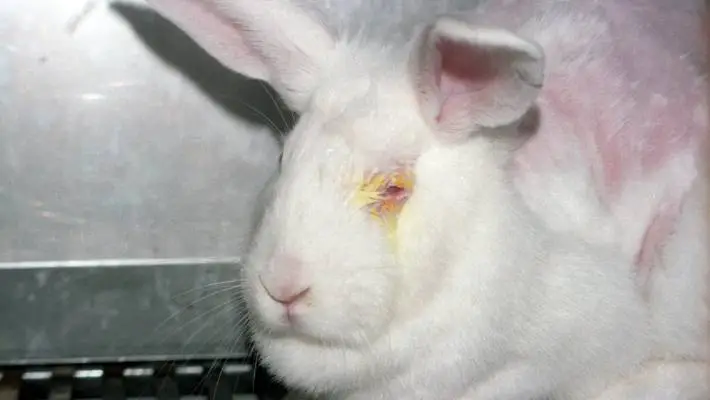
Acute Toxicity Studies – Study Design of OECD Guideline 405: Acute Eye Irritation/Corrosion Study in Rabbits Introduction The OECD Guideline 405 outlines

Acute Toxicity Studies – Study Design of OECD Guideline 420: Acute Oral Toxicity – Fixed Dose Method
Acute Toxicity Studies – Study Design of OECD Guideline 420: Acute Oral Toxicity – Fixed Dose Method Introduction The OECD Guideline 420
OECDGenetic Toxicology Studies – Study Design of Bacterial Reverse Mutation Test (Ames Test) (OECD 471) 209 Introduction The Ames test, also known
Overview of 21 CFR 117 and Food Safety Regulations Introduction Title 21 of the Code of Federal Regulations (CFR), Part 117 (commonly
Unveiling the Truth: A Deep Dive into Dietary Supplement Testing Introduction Dietary supplements have become an integral part of modern life, promising
Acute Toxicity Studies – Study Design of OECD GLP TG 404 – Acute Dermal Irritation/Corrosion Study In Rabbits Introduction The Acute Dermal
Master the Maze: Navigating the Design Control Process for Flawless Medical Devices Introduction In the world of medical devices, where even minor
How To Register Medical Devices in India? Introduction Central Drug Standard Control Organization (CDSCO) has released the Medical Devices amendment rule and classification
Critical Points in OECD GLP Study Personnel Responsibilities Introduction The Organisation for Economic Co-operation and Development (OECD) Good Laboratory Practice (GLP) Principles
Unmasking the Hidden Danger: Why Toxicological Testing Stands Guard for Medical Devices Introduction In the intricate world of medical devices, where innovative











![All Blog 49 Study Design of In Vitro Skin Sensitization - ARE-Nrf2 Luciferase Test Method [KeratinoSens] (OECD 442D)](https://www.toxlab.co/wp-content/uploads/2025/04/In-Vitro-Skin-Sensitization-ARE-Nrf2-Luciferase-Test-Method-KeratinoSens.webp)